Menu
pH Adjust 250 gm, Currently Out of Stock
Additional Information
Description
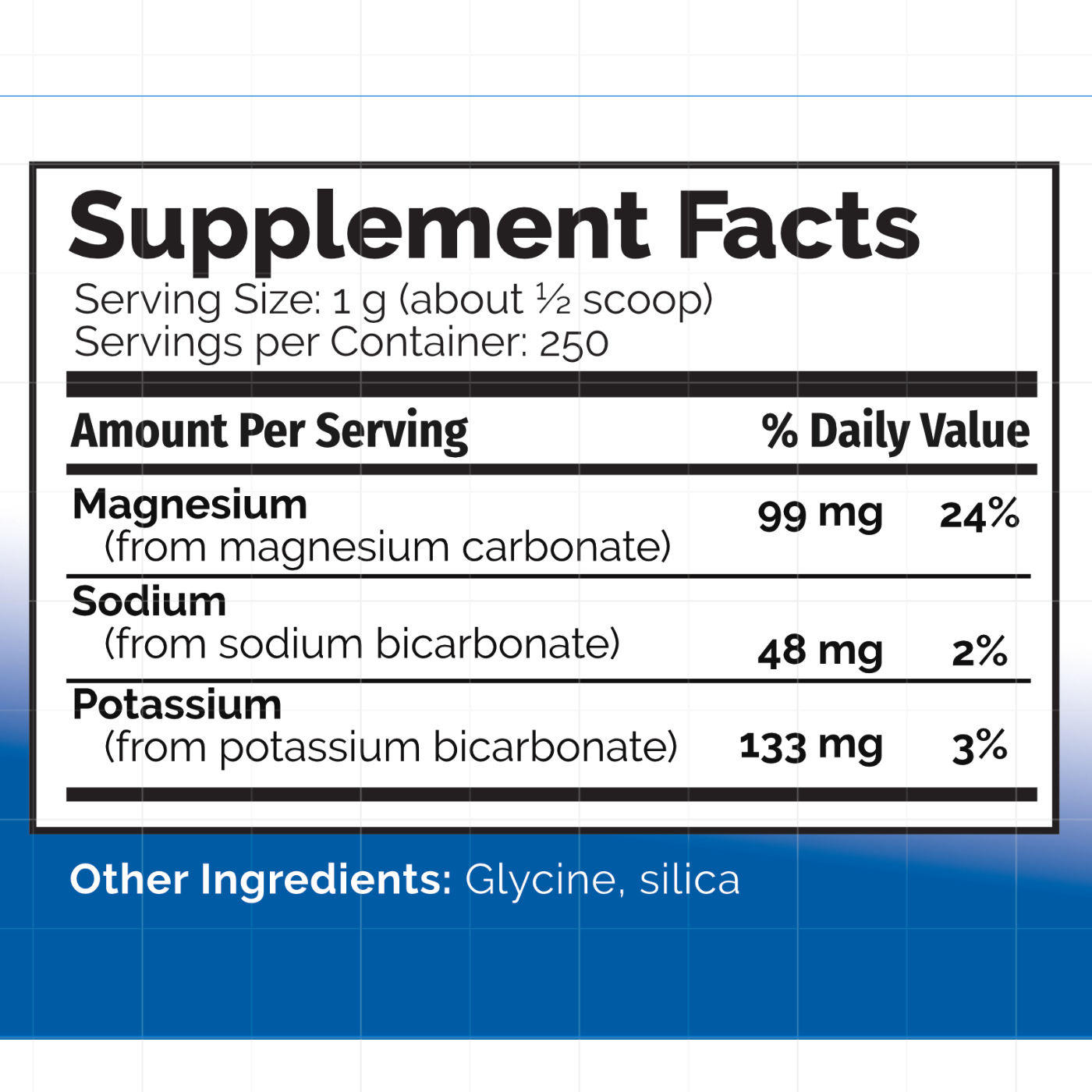
PRODUCT DESCRIPTION: pH ADJUST is designed to balance pH in the body. pH Adjust may be used to increase salivary and urinary pH; counteract overly acidic conditions in the digestive tract, blood, and kidneys; and to supplement the body with the minerals potassium, magnesium, and sodium. pH Adjust contains (in powder form) potassium bicarbonate, magnesium carbonate, sodium bicarbonate, glycine, and silica (0.5%). Each serving (about ½ tsp) contains about 350 mg of bicarbonate, 260 mg of carbonate, 133 mg of potassium, 99 mg of magnesium, 48 mg of sodium, and 59 mg of glycine. It provides a potassium to sodium ratio of 3:1.
pH ADJUST is white powder that mixes into water, juices, other liquids, and foods. It acts rapidly to raise pH levels in the body. It is easy to use, mild-tasting, and highly effective for balancing pH levels. It is gluten-free, non-GMO, vegan/vegetarian, and contains no artificial ingredients.
NUTRITIONAL CONSIDERATIONS AND APPLICATIONS: The minerals potassium, sodium, and magnesium are key substances that are involved in many important functions in the body. When combined in bicarbonates (potassium & sodium), and carbonates (magnesium), these chemicals can help to adjust and balance pH levels that are crucial to body function. pH ADJUST provides a 3:1 ratio of potassium to sodium. This ratio keeps potassium levels relatively high with respect to sodium.
The processed food diets with a high protein and low vegetable content consumed by many people in the US. and elsewhere produce conditions in the body of acidity. This in turn leads to decreased oxygenation of our cells and a greater amount of anaerobic processes in metabolism. This, in turn, leads in inadequate ATP (energy) production and the presence of unwelcome anaerobic cells and organisms.
BICARBONATE
Bicarbonate is a major element in our body. Secreted by the stomach, it is necessary for digestion. When ingested, for example, with mineral water, it helps buffer lactic acid generated during exercise and additionally reduces the acidity of dietary components. Additionally, it has a preventive effect on dental cavities. Each ½ tsp of pH ADJUST contains about 350 mg of bicarbonate.
Bicarbonate is present in all body fluids and organs and plays a major role in the acid-base balances in the human body. The first organ where food, beverages and water stay in our body is the stomach. The mucus membrane of the human stomach has 30 million glands which produce gastric juice containing not only acids, but also bicarbonate. The flow of bicarbonate in the stomach amounts from about 24 mg/hr for a basal output to about 73 mg/hr for a maximal output. Thus at least half a gram of bicarbonate is secreted daily in our stomach. This rate of gastric bicarbonate secretion is 2–10% of the maximum rate of acid secretion. In the stomach, bicarbonate participates in a mucus-bicarbonate barrier regarded as the first line of the protective and repair mechanisms. On neutralization by acid, carbon dioxide is produced from bicarbonate.
Effects of ingested bicarbonate: For digestion, bicarbonate is naturally produced by the gastric membrane in the stomach. This production will be low in alkaline conditions and will rise in response to acidity. In healthy individuals this adaptive mechanism will control the pH perfectly. To modify this pH with exogenous doses of bicarbonate, some clinical experiments have been conducted with sodium bicarbonate loads as high as 6 g. Only a transient effect on pH has been obtained. It is quite possible that bicarbonate in water may play a buffering role in the case of people sensitive to gastric acidity. Thus bicarbonate may be helpful for digestion.
The most important effect of bicarbonate ingestion is the change in acid-base balance as well as blood pH and bicarbonate concentration in biological fluids. It has been studied particularly in physically active people. Among the types of acid produced, lactic acid generated during exercise is buffered by bicarbonate. In a study on sports, a dose of 0.3 g per kg of body weight of sodium bicarbonate was given (15.25 g bicarbonate for a man of 70 kg) to subjects before performing 30 minutes cycling. While blood pH was increased and then maintained constant with this bicarbonate load due to the changes in blood bicarbonate concentrations, increased acidity and decreased bicarbonate blood concentration were observed in controlled subjects.
Prevention of renal stones: Bicarbonate also reduces the acidity of dietary components such as proteins. As an example, adding sodium or potassium bicarbonate to subjects on a high protein diet known to acidify urine and leading to hypercalciuria (high level of calcium in urine) has been shown to greatly reduce calcium urinary excretion. The effect has been observed with 5.5 g of bicarbonate supplement received daily for two weeks. A recent study highlights that a bicarbonate-rich mineral water could be useful in the prevention of the recurrence of calcium oxalate and uric acid renal stones.
Controls water absorption: many oral hydration solutions contain bicarbonate showing the usefulness of bicarbonate to control water absorption in patients at risk of dehydration.
Maintains blood pressure: Sodium intake is restricted in patients with hypertension, but it is demonstrated that the accompanying anion, such as bicarbonate, plays an important role. It is now well established that sodium bicarbonate does not raise blood pressure to the same extent as do the corresponding amounts of sodium chloride.
Decreases dental plaque: Bicarbonate has been shown to decrease dental plaque acidity induced by sucrose and its buffering capacity is important to prevent dental cavities. Other studies have shown that bicarbonate inhibits plaque formation on teeth and, in addition, increases calcium uptake by dental enamel.
CARBONATE
pH ADJUST contains about 260 mg of carbonate (and 99 mg of Mg) in the form of magnesium carbonate. Magnesium carbonate is used as an antacid that gets converted to Magnesium Chloride (MgCl) and CO2 by stomach acid. MgCl is a well absorbed form of magnesium.
MINERALS
The functions of the key minerals in pH ADJUST are described below. Each serving (about ½ tsp) of pH ADJUST contains about 350 mg of bicarbonate.
POTASSIUM
Potassium levels influence multiple physiological processes, including:
- Resting cellular-membrane potential and the propagation of action potentials in neuronal, muscular, and cardiac tissue. Due to the electrostatic and chemical properties, K ions are larger than Na ions, and ion channels and pumps in cell membranes can differentiate between the two ions, actively pumping or passively passing one of the two ions while blocking the other.
- Supports hormone secretion and action
- Improves vascular tone
- Regulates systemic blood pressure
- Increases gastrointestinal motility
- Required for acid–base homeostasis
- Supports glucose and insulin metabolism
- Plays role in mineralocorticoid action
- Supports renal concentrating ability
- Regulates fluid and electrolyte balance
MAGNESIUM
Magnesium levels influence many physiological processes and functions. These include:
- Increases energy by greater production of ATP (adenosine triphosphate) in cells
- Supports production and function of over 300 enzyme systems in the body
- Relaxes muscles / reduces muscle tension
- Boosts vitality, endurance, and strength
- Improves cardiovascular / heart health (relaxes cardiac muscle)
- Relieves pain, including chronic pain
- Ideal for arthritis / fibromyalgia / joint pain
- Improves health of skin and mucous membranes
- Eases headaches and migraine headaches
- In sports medicine — replenishes Mg levels for energy (combats fatigue, and
soothes pain and sore muscles) - Improves mood and reduces stress
- Increases memory and cognitive functions
- Boosts immune system
- Improves assimilation of calcium / builds stronger bones
- Balances calcium and magnesium levels in cells
- Proven antimicrobial and antiseptic
- Raises DHEA (dehydroepiandrosterone) levels naturally
- Eases menopause and premenstrual syndrome (pms)
- Supports healthy libido (and endocrine system)
- Anti-aging, rejuvenating, revitalizing
- Keeps cell membranes flexible
- Controls cholesterol production in the body
- Regulates blood sugar levels / needed for insulin production, transport, and
function in cells - Supports antioxidant systems
SODIUM
Sodium levels influence many physiological processes and functions. These include:
- Helps to regulate fluid levels in the human body.
- Preventing sun stroke or heat exhaustion by replacing the loss of essential electrolytes.
- Supports brain function — the brain is very sensitive to change in sodium levels of the body; deficiency of sodium often manifests as confusion and lethargy.
- Along with properly hydrating the body, it is also important to supplement one’s body with mineral-rich foods/supplements (including potassium, magnesium and sodium) to prevent muscle cramps.
- Is an important hydrating product that defends against the free radicals that accelerate the aging process.
- Helps to eliminates excess carbon dioxide in the body.
- Helps to facilitate the absorption of glucose by cells, resulting in the smooth transportation of nutrients in the body’s cell membranes.
- Supports acid/base balance by altering the proportions of acid-base alkali phosphates in the body.
- Regulates fluids by balancing the osmotic pressure in the human body
- Shares an association with chlorides and bicarbonates in maintaining a sound balance between positively charged and negatively charged ions.
COMPOSITION: One gram (about 1/2 tsp) of pH ADJUST provides the following percentages of the Daily Value
| Amount Per Serving | % Daily Value | ||
|---|---|---|---|
| Potassium (from KHCO3) Magnesium (from MgCO3) Sodium (from NaHCO3) |
133.4 mg 99.1 mg 48.1 mg |
2.8% 24.8% 2% |
|
Other ingredients: Glycine and silica (0.5%)
INGREDIENTS: Potassium bicarbonate, magnesium carbonate, sodium bicarbonate, glycine, and silica.
DIRECTIONS: As a dietary supplement, take about ½ tsp in 4–6 ounces of purified water preferably away from food, or as directed by a health care professional. Mix with a spoon and drink soon after. If you wait before drinking it’s possible that a thin layer of magnesium carbonate will settle to the bottle of the container. In this case swirl or add a bit more water, and drink right away. For extremely acidic conditions, try 4–10 doses per day, depending on acidity level. Use pH paper to ensure pH levels remain balanced. Ideal morning pH is 6.4-7.0.






 by Ohava
by Ohava